
Please refer below to an update from our healthcare analyst Dr Derek Jellinek on COVID-19 Vaccine Development:
While global containment strategies may slow the spread of Covid-19, all eyes have turned to the prospect of a vaccine, because only a vaccine can prevent people from getting sick.
Thanks in large part to early Chinese efforts to sequence and share the genetic material of Sars-CoV-2, the virus that causes Covid-19, at least 60 vaccine candidates are in development via companies and academic institutions.
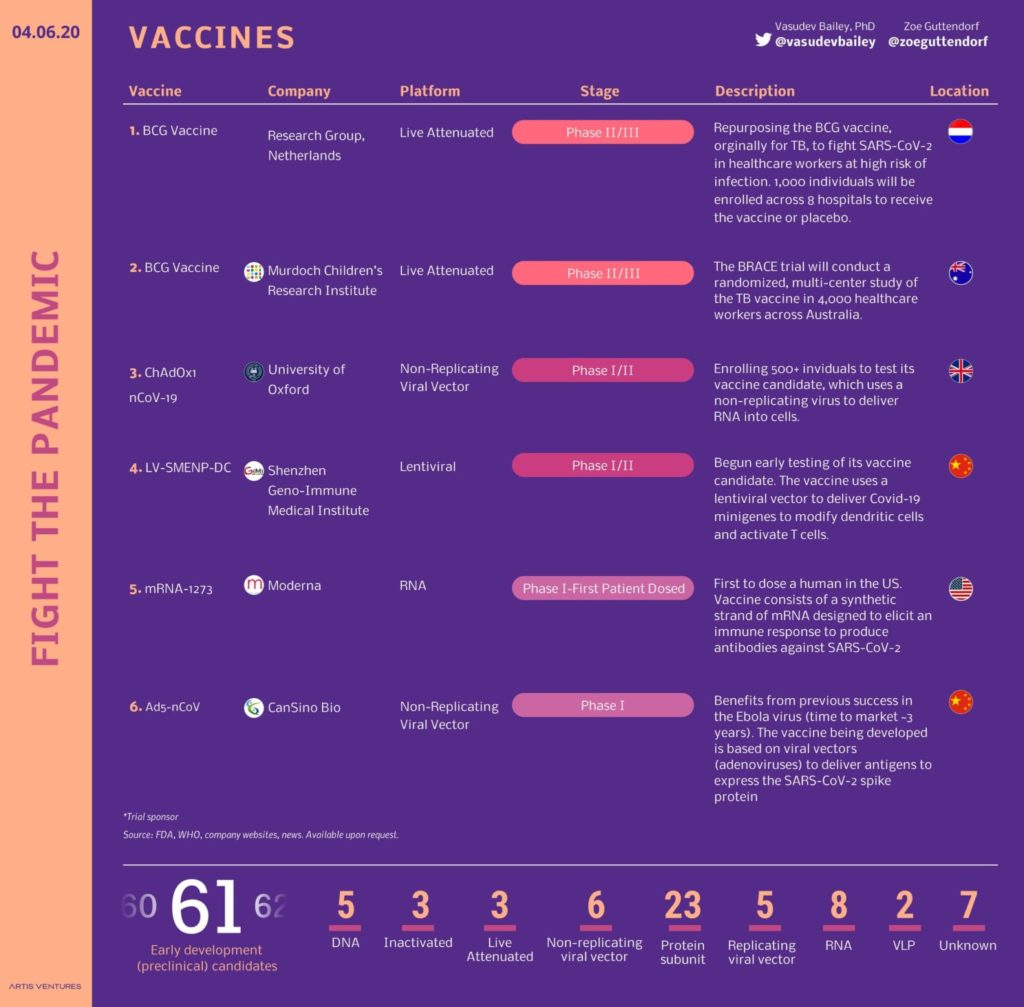
While the speed with which vaccine candidates have been produced is unprecedented, any likely stumbles will happen in clinical or human trials. These trials are a required perquisite to regulatory approval and usually take place in 3 phases:
Phase 1 - involving a few dozen healthy volunteers, tests the vaccine for safety, monitoring for adverse effects;
Phase 2 - involving several hundred people, usually in a part of the world affected by the disease, looks at how effective the vaccine is, and
Phase 3 - does the same in as in Phase 2, but in several thousand people.
So why all of this testing? Well, trials essentially screen out the 'duds', those candidates that are either unsafe or ineffective or both, which is why clinical trials can't be skipped or hurried. However, approval can be accelerated, especially if regulatory have approved similar products before. But we note, Sars-CoV-2 is a novel pathogen in humans and many of the technologies being used to build vaccines are relatively untested too. So we wouldn't expect any vaccine to be commercialised inside 12 months.
Beyond approval, other potential issues to think about include production capacity (with many of the organisations in the Covid-19 vaccine race simply not having adequate capacity) and global distribution (essentially, a political game to initiate a global immunisation programme).
As all of this is being debated, the pandemic will probably have peaked and declined before a vaccine is available. Nevertheless, a vaccine could still save many lives, especially if the virus becomes endemic or perennially circulating - like flu - and there are further, possibly seasonal, outbreaks...as we believe.
But until then, our best hope is to contain the disease as far as possible. To repeat the sage advice: wash your hands frequently with soap and water; cover your cough and sneeze; and if unwell, avoid contact with others.
This article is not to be tampered with, altered or changed in any way without the prior consent of the author. The Authors noted in this email may own or have traded or intend to trade in the shares discussed above. This information was prepared as a private communication and is not intended for public circulation, publication or use by any third party. The information contained in this report is provided to you by Morgans Financial Limited as general advice only, and is made without consideration of an individual's relevant personal circumstances. Morgans Financial Limited ABN 49 010 669 726, its related bodies corporate, directors and officers, employees, authorised representatives and agents (“Morgans”) do not accept any liability for any loss or damage arising from or in connection with any action taken or not taken on the basis of information contained in this report, or for any errors or omissions contained within. It is recommended that any persons who wish to act upon this report consult with their investment adviser before doing so.